
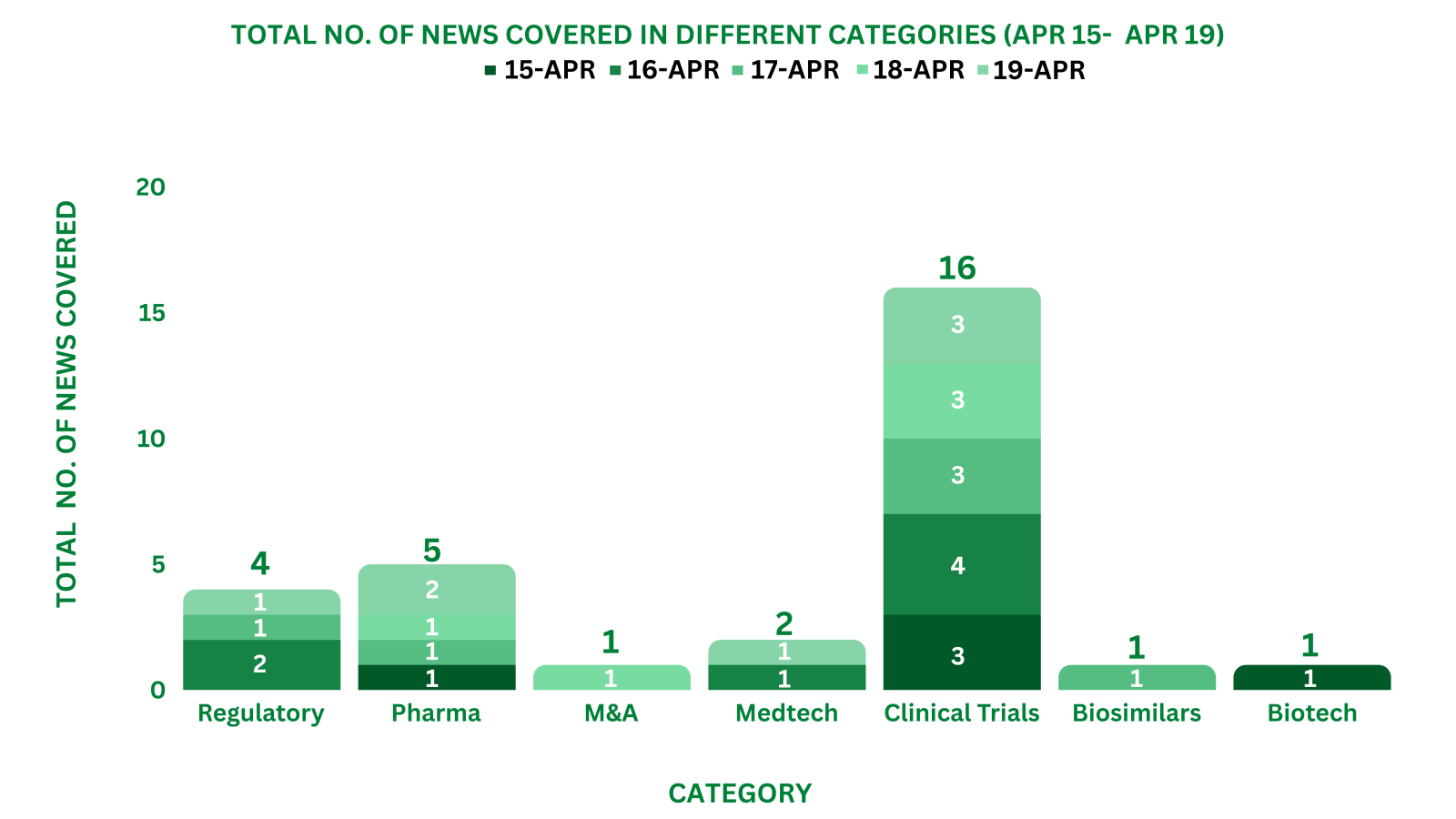
PharmaShots Weekly Snapshots (April 15 – April 19, 2024)
This week PharmaShots’ news was all about the updates on Regulatory, Clinical Trials, Pharma, Animal Health & MedTech.
Check out our full report below:

Regulatory
The US FDA Accepts GSK’s BLA of Meningococcal ABCWY Vaccine for Invasive Meningococcal Disease (IMD)
Read More: GSK
NovelMed’s NM5072 Receives the US FDA’s Orphan Drug Designation to Treat Paroxysmal Nocturnal Hemoglobinuria (PNH)
Read More: NovelMed
Sentynl Therapeutics’ Nulibry (fosdenopterin) Gains the MHRA’s Approval to Treat Molybdenum Cofactor Deficiency (MoCD) Type A
Read More: Sentynl Therapeutics
Takeda’s Entyvio Receives the US FDA’s Approval for Subcutaneous Administration to Treat Moderately to Severely Active Crohn’s Disease (CD)
Read More: Takeda

Nxera Pharma Collaborates with Handok to Commercialize Pivlaz in South Korea
Read More: Nxera Pharma & Handok
Medincell Collaborates with AbbVie for the Development of Therapies Across Various Indications
Read More: Medincell & AbbVie
IGM Biosciences Revises its Collaboration with Sanofi
Read More: IGM Biosciences & Sanofi
GV20 Therapeutics Collaborates with Merck to Evaluate GV20-0251 + KEYTRUDA in Patients with Advanced Solid Tumors
Read More: GV20 Therapeutics & Merck
Alvotech and Teva Pharmaceuticals Collaborates to Expand Access for Adalimumab-ryvk (Interchangeable Biosimilar, Humaira) in US
Read More: Alvotech & Teva

Essential Pharma Acquires Reminyl (galantamine hydrobromide) Oral Capsules from Janssen
Read More: Essential Pharma & Reminyl

TELA Bio Launches OviTex IHR to Repair Inguinal Hernia Across the US
Read More: TELA Bio
Shinobi Therapeutics Teams Up with Panasonic to Develop Affordable and Efficient iPS Cell Therapy Manufacturing Technology
Read More: Shinobi Therapeutics & Panasonic

AbbVie Reports Data from the P-III Trial of Atogepant as a Preventive Treatment of Migraine
Read More: AbbVie
Roche Reports Data from the P-III Clinical Evaluation of Columvi to Treat R/R Diffuse Large B-cell Lymphoma
Read More: Roche
MaaT Pharma to Highlight Data from the Early Access Program of MaaT013 to Treat Acute Graft-Versus-Host Disease (aGvHD) at EBMT 2024
Read More: MaaT Pharma
Novartis Reports Data from the P-III Study of Fabhalta (iptacopan) for the Treatment of IgA Nephropathy (IgAN)
Read More: Novartis
ORIC Pharmaceuticals Doses First Patients Across Expansion Arms in the P-Ib Study of ORIC-114 for the Treatment of Mutated NSCLC
Read More: ORIC Pharmaceuticals
Corcept Concludes Patient Recruitment in the P-II Study of Dazucorilant for Amyotrophic Lateral Sclerosis (ALS)
Read More: Corcept
AstraZeneca Reports Results from the P-III (TOPAZ-1) Study of Imfinzi for Treating Advanced Biliary Tract Cancer
Read More: AstraZeneca
Sanofi to Highlight New Data from the P-II Study of Frexalimab for the Treatment of Multiple Sclerosis at AAN 2024
Read More: Sanofi
GSK to Feature Results from the P-III (ZOSTER-049) Study of Shingrix for Protection Against Shingles at ESCMID Global 2024
Read More: GSK
Roche to Highlight the P-III (OCARINA II) Trial Results of Ocrevus for Treating Progressive and Relapsing Multiple Sclerosis at AAN 2024
Read More: Roche
GSK to Highlight Results from the P-III (EAGLE-1) Study of Gepotidacin for Treating Uncomplicated Urogenital Gonorrhoea (GC) at ESCMID 2024
Read More: GSK
Lilly Reports the P-III (SURMOUNT-OSA) Study Data of Tirzepatide in Patients with Obstructive Sleep Apnea (OSA) and Obesity
Read More: Eli Lilly
Artiva Biotherapeutics Doses First Patient with AlloNK in the P-I Study for Treating Lupus Nephritis
Read More: Artiva
Cerevel Therapeutics Reports Results from P-III Adjunctive Study Evaluating Tavapadon for the Treatment of Advanced Parkinson’s Disease
Read More: Cerevel
Oncternal Therapeutics Reports First Patient Dosing in Fourth Cohort of P-I/II Study of ONCT-534 for the Treatment of R/R mCRPC
Read more: Oncternal Therapeutics
Ventus Therapeutics Reports Results from the P-I Study of VENT-02 in Healthy Volunteers
Read more: Ventus Therapeutics

Teva and Alvotech’s Selarsdi (Biosimilar, Stelara) Receives the US FDA’s Approval
Read More: Teva Pharmaceuticals

Seismic Therapeutic Highlights Preclinical Data of S-1117 for the Treatment of Autoimmune Disorders at AAN 2024
Read More: Seismic Therapeutic
Related Post:- PharmaShots Weekly Snapshots (April 08 – April 12, 2024)
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.













